Building a cycle-stable sulphur cathode by tailoring its redox reaction into a solid-phase conversion mechanism†
Abstract
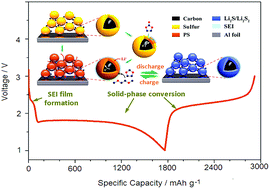
Sulphur has been actively investigated as a high capacity, naturally abundant and low cost cathode for next generation rechargeable lithium batteries. However, the poor cyclability and low capacity utilization of sulphur materials remain a great challenge for battery applications. To overcome this problem, we proposed a new strategy to convert the redox chemistry of sulphur cathodes from the dissolution–deposition mechanism to a solid-phase conversion (SPC) reaction by in situ formation of a thin and compact solid electrolyte interface (SEI) on the sulphur surface through a prompt nucleophilic reaction of soluble polysulfide intermediates with carbonate molecules specially designed as a co-solvent in the electrolyte. The such-formed SEI film can effectively block the penetration of the electrolyte but allows Li+ transport for electrochemical conversion, thus completely suppressing the generation and dissolution of polysulfide intermediates without sacrificing the two-electron redox capacity of sulphur. As a result, the S/C cathode in a VC/DME + DOL co-solvent electrolyte demonstrated a stable cycling capacity of ∼1100 mA h g−1 and a high capacity retention of 88% over 400 cycles with a coulombic efficiency of ∼100%, showing a prospect for battery application. More significantly, the strategy and method developed in this work may provide a new insight for future development of structurally and electrochemically stable sulphur cathodes for building practically viable Li–S batteries.


 Please wait while we load your content...
Please wait while we load your content...