Organic heterojunction photocathodes for optimized photoelectrochemical hydrogen peroxide production†
Abstract
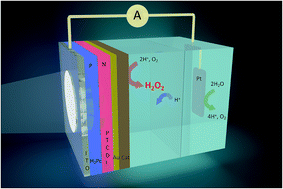
Solar-to-chemical conversion of sunlight into hydrogen peroxide as a chemical fuel is an emerging carbon-free sustainable energy strategy. The process is based on the reduction of dissolved oxygen to hydrogen peroxide. Only limited amounts of photoelectrode materials have been successfully explored for photoelectrochemical production of hydrogen peroxide. Herein we detail approaches to produce robust organic semiconductor photocathodes for peroxide evolution. They are based on evaporated donor–acceptor heterojunctions between phthalocyanine and tetracarboxylic perylenediimide, respectively. These small molecules form nanocrystalline films with good operational stability and high surface area. We discuss critical parameters which allow fabrication of efficient devices. These photocathodes can support continuous generation of high concentrations of peroxide with faradaic efficiency remaining at around 70%. We find that an advantage of the evaporated heterojunctions is that they can be readily vertically stacked to produce tandem cells which produce higher voltages. This feature is desirable for fabricating two-electrode photoelectrochemical cells. Overall, the photocathodes presented here have the highest performance reported to date in terms of photocurrent for peroxide production. These results offer a viable method for peroxide photosynthesis and provide a roadmap of strategies that can be used to produce photoelectrodes with even higher efficiency and productivity.


 Please wait while we load your content...
Please wait while we load your content...