Mechanistic insight into dendrite–SEI interactions for lithium metal electrodes†
Abstract
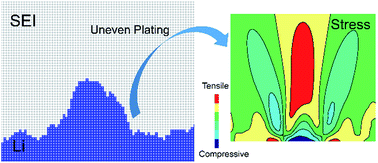
The stability and homogeneity of the solid electrolyte interphase (SEI) layer are critical toward understanding the root causes behind performance decay and safety concerns with lithium metal electrodes for energy storage. This study focuses on deducing mechanistic insights into the complexations between the Li metal electrode and SEI during electrodeposition. It is found that the formation of Li dendrite can be initiated by two distinct mechanisms: (i) aggressive Li-ion depletion near the anode–SEI interface at high reaction rates or low temperature attributed to transport limitations, and (ii) spatially varying reaction kinetics at the SEI–electrode interface due to SEI inhomogeneity even at low currents. Subsequent mechanical stability analyses reveal that significantly high stress is generated due to nonuniform Li electrodeposition which could lead to crack formation in the existing SEI layer, and consequently exposure of fresh lithium to the electrolyte resulting in enhanced capacity fading. Furthermore, a non-dimensional analysis relating the interfacial stress induced failure propensity to electrochemical Biot number and SEI heterogeneity factor is proposed, which delineates stable lithium deposition regimes.


 Please wait while we load your content...
Please wait while we load your content...