IrOOH nanosheets as acid stable electrocatalysts for the oxygen evolution reaction†
Abstract
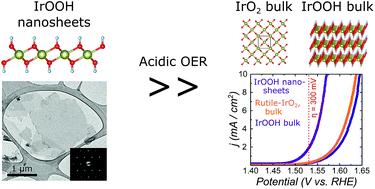
In solids, heterogeneous catalysis is inherently bound to reactions on the surface. Yet, atomically efficient preparation of specific surfaces and the characterization of their properties are impeding its applications towards a clean energy future. Here, we present the synthesis of single layered IrOOH nanosheets and investigations of their structure as well as their electrochemical properties towards oxygen evolution under aqueous acidic conditions. The nanosheets are synthesized by treating bulk IrOOH with a tetrabutylammonium hydroxide solution and subsequent washing. Electron diffraction shows that the triangular arrangement of the edge sharing Ir(O,OH)6 octahedra found in the layers of bulk IrOOH is retained after exfoliation into single layers. When incorporated as an active component in Ti electrodes, the nanosheets exhibit a Tafel slope of 58(3) mV dec−1 and an overpotential of η10 mA cm−2 = 344(7) mV in 0.1 M HClO4, while retaining the trivalent oxidation state of iridium. They outperform bulk rutile-IrO2 and bulk IrOOH as electrocatalytic water oxidation catalysts under the same conditions. The results of this study on the structure–property relationships of low valence IrOOH nanosheets offer new pathways for the development of atom efficient, robust and highly active oxygen evolution catalysts.


 Please wait while we load your content...
Please wait while we load your content...