Suppressing the voltage decay of low-cost P2-type iron-based cathode materials for sodium-ion batteries†
Abstract
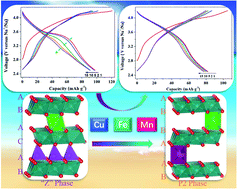
Rechargeable sodium-ion batteries with earth-abundant Fe/Mn based cathodes are a promising choice for grid-scale applications. However, the key candidate, P2-type Fe-based materials, suffers from severe voltage decay during battery operation due to Fe3+ migration to the neighboring tetrahedral sites. In this study, two Fe-based layered oxides, Na0.7[Cu0.15Fe0.3Mn0.55]O2 and Na0.7[Cu0.2Fe0.2Mn0.6]O2, were prepared. With a combination of in situ XRD, X-ray PDF, and hard and soft X-ray absorption, we demonstrate that the voltage decay in Fe-based layered oxides has dynamic origins. Drastic phase transition can be triggered by higher upper voltage limit, while partially irreversible Fe migration leads to voltage fading. With excess Cu doped into the crystal lattice, Fe migration can be considerably mitigated and therefore, structural stability can be maintained. Furthermore, Cu introduction brings about extra capacity via the correlation between transition metal elements and ligand oxygen, which may well compensate for capacity loss from inert impurity doping. Possible strategies for suppressing the detrimental voltage decay in battery cathodes can be proposed accordingly.


 Please wait while we load your content...
Please wait while we load your content...