Self-assembly optimization of cadmium/molybdenum sulfide hybrids by cation coordination competition toward extraordinarily efficient photocatalytic hydrogen evolution†
Abstract
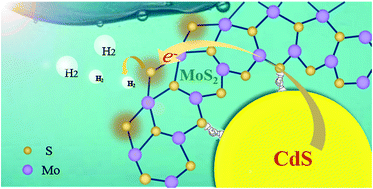
Combining photoharvesting semiconductors with MoS2 co-catalysts is an intriguing approach to develop inexpensive and efficient photocatalysts for visible-light-driven hydrogen (H2) evolution; however, how to coordinate light absorbers and catalytic sites still remains a great challenge. Here, we propose a facile strategy for optimizing assembly of CdS–MoS2 (CM) nanohybrids with controlled active edge exposure by cation coordination competition in one-pot solvothermal synthesis. With the involvement of Cd ions, more formed CdS nanocrystals coordinated preferentially with MoS2 edges self-optimize into unique CM hybrids, which enables the maximum performances of active area and photo-induced electron transfer and injection into the photocatalytic H2 evolution reaction (HER). As a result, the optimum CM hybrids exhibit an outstanding and stable photocatalytic activity with a H2 evolution rate of up to 1009 mmol h−1 g−1, more than 104-fold higher than that of pure CdS, which is the best among the state-of-the-art heterogeneous photocatalysts. This work provides a facile strategy for a controlled configuration of MoS2 edge active sites on photoharvesting semiconductors toward high-efficiency solar photocatalytic H2 generation.


 Please wait while we load your content...
Please wait while we load your content...