Electroreduction of a CoII coordination complex producing a metal–organic film with high performance toward electrocatalytic hydrogen evolution†
Abstract
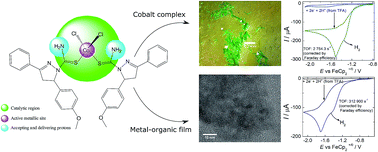
This paper describes the synthesis and structural characterization of a novel, cheap and simple CoII complex (CoII(L)2Cl2) based on the 1,3,5-trisubstituted-pyrazoline ligand along with the electrochemical production of metal–organic electroactive films derived from this new complex. These systems were applied as electrocatalysts for hydrogen production (ACN/ACA or ACN/TFA medium) where both materials presented high performance toward hydrogen evolution. Compared to the CoII complex, the electroactive films exhibited significant electroactivity toward hydrogen evolution, presenting a remarkable TOF for H2 production (312 900 s−1, corrected by Faraday efficiency) in the presence of TFA. In addition, the generated metal–organic film showed high stability toward the electrocatalytic hydrogen production, supporting at least 1000 cycles at 20 mV s−1 in the large potential range investigated, as well as good performance and stability in the presence of 0.5 M H2SO4. Relevant insights into the mechanistic details and the role played by the CoII complex and the films during the catalytic hydrogen production are also discussed in light of the structural features and electrochemical experiments.


 Please wait while we load your content...
Please wait while we load your content...