Mesoporous carbon matrix confinement synthesis of ultrasmall WO3 nanocrystals for lithium ion batteries†
Abstract
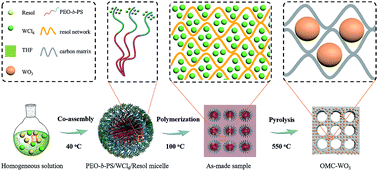
Transition metal oxides (TMOs)/carbon nanocomposites are promising for high capacity long life lithium ion batteries (LIBs). Herein, we report a mesoporous carbon matrix confinement growth strategy to synthesize ultrasmall WO3 nanocrystals for lithium storage. In this strategy, WCl6 and phenolic resins (resol) are co-assembled with amphiphilic diblock copolymer PEO-b-PS into ordered mesostructures through an evaporation induced self-assembly (EISA) process. During the pyrolysis process, the resol molecules can be polymerized and carbonized into amorphous mesoporous carbon matrices, which lock the amorphous W species well. Then, WO3 nanocrystals are formed and are uniformly distributed in the ordered mesoporous carbon matrix with the increased pyrolysis temperature; moreover, the particle size is well controlled to ∼3 nm under the confinement effect of the carbon matrices. The resultant ordered mesoporous carbon/WO3 composites show very large pore size (∼11.3 nm), high surface area (∼157 m2 g−1), high pore volume (∼0.25 cm3 g−1), and WO3 content of 84%. As an anode material for LIBs, the obtained composites show excellent cycling stability and rate performance. A high specific capacity of 440 mA h g−1 can be achieved after 100 cycles at a current density of 0.1 A g−1. We believe that such a confinement synthesis strategy is versatile to create TMO-based nanocomposites for outstanding LIBs.


 Please wait while we load your content...
Please wait while we load your content...