An air-stable lithiated cathode material based on a 1,4-benzenedisulfonate backbone for organic Li-ion batteries†
Abstract
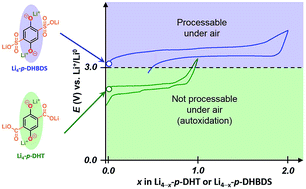
To meet current market demands as well as emerging environmental concerns there is a need to develop less polluting battery technologies. Organic electrode materials could offer the possibility of preparing electrode materials from naturally more abundant elements and eco-friendly processes coupled with simplified recycling management. However, the potential use of organic electrode materials for energy storage is still challenging and a lot of developments remain to be achieved. For instance, promoting high-energy Li-ion organic batteries inevitably requires the development of lithiated organic electrode materials which are able to be charged (delithiated) at a high enough potential (>3 V vs. Li+/Li0) – a challenging point rarely discussed in the literature. Here, we evaluate tetralithium 2,5-dihydroxy-1,4-benzenedisulfonate as an air-stable lithiated cathode material for the first time and its reversible Li+ electrochemical extraction. Quite interestingly, in comparison with the dicarboxylate counterpart, it was observed that the theoretical two-electron reaction is readily reached with this organic structure and at an average operating potential of 650 mV higher.


 Please wait while we load your content...
Please wait while we load your content...