Rhodium metal–rhodium oxide (Rh–Rh2O3) nanostructures with Pt-like or better activity towards hydrogen evolution and oxidation reactions (HER, HOR) in acid and base: correlating its HOR/HER activity with hydrogen binding energy and oxophilicity of the catalyst†
Abstract
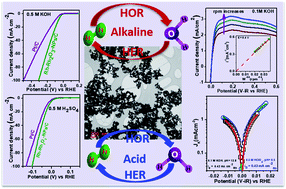
Design and synthesis of HER/HOR catalysts are of crucial importance for the development of polymer fuel cells and water electrolyzers. We report the synthesis of Rh–Rh2O3 nanoparticles/nitrogen-doped carbon composite (Rh–Rh2O3-NPs/C) for HER/HOR applications. The HER activity of this catalyst is ∼2.2 times and 1.43 times better than that of commercial Pt/C in base and acid, respectively. Rh–Rh2O3-NPs/C exhibited 10 mA cm−2 current density at an overpotential of 63 mV and 13 mV with Tafel slopes of 70 mV dec−1 and 32 mV dec−1 in base and acid, respectively. The catalyst showed superior HOR activity at all pH values. The exchange current densities were ∼0.425 mA cmRh−2 and ∼0.43 mA cmRh−2 in base and acid, respectively. In base, the HOR and HER activities of Rh–Rh2O3-NPs/C are 50-fold and 10-fold higher, respectively, in comparison with those of the Rh2O3-free RhNPs/C catalyst, although the HER/HOR activity of both the catalysts is comparable in acid. In base, the adsorption of OH− species (OHads) on Rh2O3 sites increases the reactivity of hydrogen intermediate (Habs), which leads to the enhancement of HOR activity of Rh–Rh2O3-NPs/C. For HER in base, the adsorptive dissociation of water occurs on the Rh2O3 sites to form Hads on the neighboring Rh sites and then, the recombination of Hads results in the formation of hydrogen molecules. This study may provide an opportunity to develop an efficient catalyst for hydrogen-based renewable energy technologies.


 Please wait while we load your content...
Please wait while we load your content...