Interfacial proton enrichment enhances proton-coupled electrocatalytic reactions†
Abstract
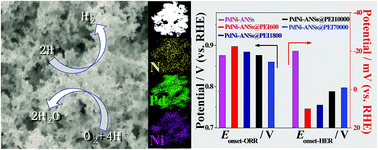
Proton-coupled electrocatalytic reactions, such as the oxygen reduction reaction (ORR) and hydrogen evolution reaction (HER) in acidic media, are very important half-reactions of fuel cells and water electrolysis. Aiming at increasing the interfacial proton concentration to improve the ORR/HER activity, we synthesize PdNi alloy nanostructures (PdNi-ANSs)–polyethyleneimine (PEI) inorganic–organic nanocomposites (PdNi-ANSs@PEI) by a PEI assisted cyanogel-reduction strategy. Various physical characterization techniques show that PdNi-ANSs are successfully functionalized by the PEI polymer during the synthesis of PdNi-ANSs@PEI because of the N–Pd interaction. Thanks to the increased interfacial proton concentration caused by the protonation of amine groups at PEI in acidic media, the molecular weight optimized PdNi-ANSs@PEI exhibit enhanced ORR/HER activity compared to PdNi-ANSs without PEI and commercial electrocatalysts, which indicates that the electrocatalytic activity of metal nanostructures can be remarkably improved by reasonable interface design.


 Please wait while we load your content...
Please wait while we load your content...