Phase transformation, ionic diffusion, and charge transfer mechanisms of KVOPO4 in potassium ion batteries: first-principles calculations†
Abstract
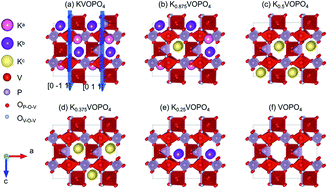
First-principles calculations based on density functional theory were performed to investigate the electrochemical properties of K1−xVOPO4 in potassium-ion batteries (KIBs). The material showed multiple phase transitions during K ion extraction, which began with a two-phase transition (0 ≤ x ≤ 0.5), followed by a solid-solution transition (0.5 < x ≤ 0.625), another two-phase transition (0.625 < x ≤ 0.75), and finally a solid-solution transition (0.75 < x ≤ 1). These processes resulted in a small total unit cell volume variation of 6.6%, which was beneficial for the cycle stability of KIBs. Density of states and Bader charge analysis revealed that both V and O participated in the charge transfer process, where V acted as the redox center of KVOPO4 contributing to the K storage capacity, and O acted as a charge transfer medium between V and K. The stepwise increased repulsion between V cations caused three voltage plateaus for K1−xVOPO4. In addition, the one-dimensional diffusion pathway for K ions with low energy barriers of 0.214–0.491 eV ensured high K ion mobility resulting in superior high rate capability.


 Please wait while we load your content...
Please wait while we load your content...