Synergistic core–shell interactions enable ultra-low overpotentials for enhanced CO2 electro-reduction activity†
Abstract
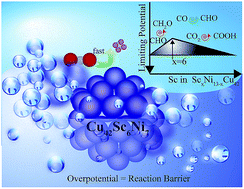
Carbon dioxide is a major culprit of climate change, dramatically warming the Earth with its accumulation in the atmosphere. Converting CO2 to high end fuels could potentially solve environmental problems and create a sustainable energy cycle. In spite of Cu being the most successful catalyst capable of producing hydrocarbons from CO2, its efficiency suffers from high overpotentials, which originate from the highly endothermic CO protonation to CHO. Using density functional theory (DFT), we optimize the composition of a bimetallic core TMxNi13−x (TM = 3d transition metals; x = 3, 6, and 9) encapsulated in a 42 atom Cu icosahedral shell to lower the CO2 reduction overpotential. On Sc5(6)Ni8(7)–Cu42, this overpotential reduces to an unprecedented value of ∼0.17 V below the standard potential value (SPV). These specific core compositions optimize the position of the d-band center of the shell, required for better interaction with CHO π orbitals than CO, giving exergonic CO reduction. The estimated statistical coverage of CHO exceeds 60% under ambient conditions on Sc6Ni7–Cu42, which will promote CO2 conversion to the high end fuel CH4 without CO poisoning of the catalyst. The effect of implicit solvent is found to be marginal on the stability, reaction barriers and overpotentials, ensuring the robustness of Sc5(6)Ni8(7)–Cu42 for efficient CO2 electro-reduction.


 Please wait while we load your content...
Please wait while we load your content...