Establishing new scaling relations on two-dimensional MXenes for CO2 electroreduction†
Abstract
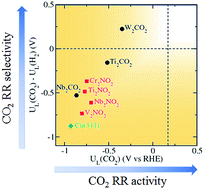
Electrochemical reduction of CO2 enables the utilisation and conversion of CO2 greenhouse gas into valuable fuels and chemicals. Transition metals, copper in particular, are most widely investigated as catalysts due to their ability to convert CO2 into a multitude of value-added carbon products. However, linear scaling relations prevent similarly-bound reaction intermediates (*CO, *CHO) from being stabilised independently on the surface, therefore severely limiting the activity and overpotential of transition metal catalysts. Here, we present a theoretical study of two-dimensional transition metal carbides and nitrides (MXenes) as promising electrocatalysts for the reduction of CO2 to CH4. A different CO2 reduction pathway through –H coordinated *HCOOH was discovered on O-terminated MXene catalysts due to generally weaker *CO binding. New scaling relations were established based on the alternating –C and –H coordination of the intermediates along the minimum energy pathway. Importantly, we found that the limiting potential of the MXene catalysts is determined by the binding energies of *COOH and/or *HCOOH which can be tuned independently, allowing significantly lower overpotential to be achieved compared to transition metals. In particular, two promising MXenes with theoretical overpotentials of 0.52 and 0.69 V, and competitive selectivity with respect to hydrogen evolution, were identified.
- This article is part of the themed collection: Journal of Materials Chemistry A Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...