Energetic salts of 4-nitramino-3-(5-dinitromethyl-1,2,4-oxadiazolyl)-furazan: powerful alliance towards good thermal stability and high performance†
Abstract
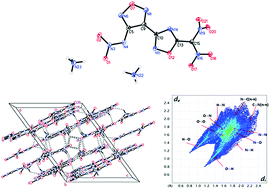
Two different methods were developed to synthesize 4-amino-3-(5-acetic acid methyl ester-1,2,4-oxadiazolyl)-furazan (1), the precursor to prepare 4-nitramino-3-(5-dinitromethyl-1,2,4-oxadiazolyl)-furazanate, in scalable good yields. In four steps, three energetic salts were obtained with an overall yield of ∼50% and show promising characteristics of typical secondary explosives. The structure of the diammonium salt (2a) was confirmed by X-ray single crystal diffraction. It has a high crystal density of 1.840 g cm−3 at 150 K and a measured density of 1.804 g cm−3 at room temperature. All of the compounds were fully characterized by infrared spectroscopy (IR), 1H NMR, and 13C NMR spectroscopy as well as elemental analysis. Compound 2b and 2c exhibit better sensitivities and comparable detonation properties relative to HMX, showing great promise as replacements.


 Please wait while we load your content...
Please wait while we load your content...