Role of the Mn substituent in Na3V2(PO4)3 for high-rate sodium storage†
Abstract
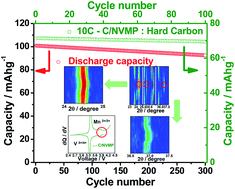
Mn-doped Na3V2−xMnx(PO4)3 (x = 0 and 0.1) cathode materials, which allow extraction of 2 mol of Na from the formula unit, were synthesized, and the effect of doping was investigated. A large discharge capacity of 114 mA h g−1, approaching 96.6% of the theoretical capacity, was attained, which was theoretically activated by the V3+/4+ redox reaction. Structural investigation was performed using Rietveld refinement of X-ray diffraction data and X-ray absorption near-edge structure analysis. Successful substitution of Mn at the V site reduced the band gap energy from 3.32 to 2.44 eV according to density functional theory calculations and resulted in improved electron transfer. In addition, surface modification by carbon led to a high electrical conductivity of up to 10−3 S cm−1. The improved electrical conductivity resulted in a high discharge (reduction) capacity of approximately 114 mA h g−1 at a current of 10 mA g−1 (at 0.1C) with an excellent retention of approximately 108 mA h g−1 after the 100th cycle. The electrode provided a stable discharge capacity over 50 cycles tested at 10C (78 mA h g−1, 90% retention) and of 64 mA h g−1 even at 50C (5 A g−1). The synergistic effect of high electrical conductivity by carbon coating and reduction of the bandgap by Mn doping led to the delivery of a capacity of approximately 62 mA h g−1 (at 1C) at −10 °C. In addition, the C/Na3V1.9Mn0.1(PO4)3 cathode was fabricated as a full cell using a hard carbon anode to determine the above synergetic effects. Superior electrode performance was achieved, with an initial discharge capacity of 104 mA h g−1 and 92% capacity retention versus the initial capacity after 300 cycles.


 Please wait while we load your content...
Please wait while we load your content...