High-performance Na ion cathodes based on the ubiquitous and reversible O redox reaction†
Abstract
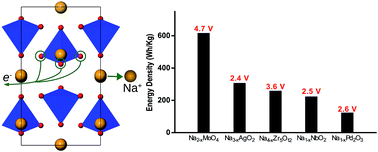
Utilising reversible oxygen redox in Na and transition metal oxides offers unprecedented opportunities for the design of high voltage, high capacity and affordable cathodes for application in rechargeable Na-ion batteries. Through a judicious materials search and theoretical investigations, we identified new compounds with excellent energy storage properties that rely on oxygen states for charge compensation during the redox reaction. According to our predictions, Na2−xMoO4 demonstrates a voltage of 4.743 V and an energy density of ∼617.3 W h kg−1. These values exceed the performance of most commercialised Na-ion cathode materials. Furthermore, both Na4−xZr5O12, demonstrating a voltage of 3.583 V, and Na1−xPd2PO3, demonstrating a voltage of 2.630 V, exhibit a meagre absolute volume change of ∼1% during the sodiation/desodiation process. Because of this minor volume change, these compounds are suitable for all-solid-state battery applications. An examination of the electronic structures of these compounds reveals that O states are always present at the top of the valence band regardless of the presence of 4d transition metal species or their oxidation states. This feature is attributed to the exceedingly substantial 4d–2p hybridisation over the entire valence band which also prevents the bonding of oxidised O ions in the desodiated compounds, thus preventing irreversible oxygen loss.


 Please wait while we load your content...
Please wait while we load your content...