Na0.97KFe(SO4)2: an iron-based sulfate cathode material with outstanding cyclability and power capability for Na-ion batteries†
Abstract
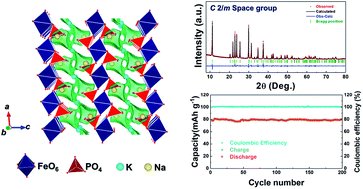
In this work, a novel cathode material for Na-ion batteries, Na0.97KFe(SO4)2, was successfully prepared via slow evaporation and a low-temperature process, and its outstanding electrochemical performance was demonstrated. Based on the structural information of KFe(SO4)2 obtained from Rietveld refinement of X-ray diffraction data, the possible atomic sites for Na ions entering the structure were verified using bond-valence sum energy maps. Electrochemical measurements and X-ray absorption near-edge structure analyses revealed that ∼0.97 mol Na ions can be reversibly (de)intercalated into the structure via the Fe3+/Fe2+ redox reaction. The average redox potential of Na0.97KFe(SO4)2 was shown to be ∼3.27 V (vs. Na+/Na), which is higher than that of other Fe-based phosphates owing to the inductive effect of (SO4)2−. It was verified that the specific capacity of Na0.97KFe(SO4)2 at C/10 was ∼85 mA h g−1. At 10C (full charge/discharge in 12 min), ∼53% of its capacity measured at C/10 was retained. In addition, up to ∼99% of its initial capacity was retained over 200 cycles at 1C with a high coulombic efficiency of 99.3097%, which was attributed to the negligible volume change (∼1.8%) during charge/discharge.


 Please wait while we load your content...
Please wait while we load your content...