From monometallic Au nanowires to trimetallic AuPtRh nanowires: interface control for the formic acid electrooxidation†
Abstract
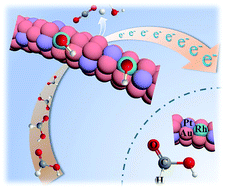
Developing an easy-to-expand synthetic method for nanostructures is highly desired, which will help in investigating the effect of the type and content of the extended component on catalytic performance. Based on original Au nanowires (NWs), we design a two-step interface control strategy to synthesize a series of bimetallic AuPt NWs and trimetallic AuPtRh NWs for the formic acid oxidation reaction (FAOR). By controlling the proportion of Au and Pt, a Au/Pt interface can achieve a AuPt alloy structure, which makes Au6Pt1/C show a complete direct dehydrogenation pathway for the FAOR. The further design and fabrication of an advanced Au/Pt/Rh interface are proved to be more active than the Au/Pt interface for the FAOR. The mechanism analysis and experimental results demonstrate that Rh atoms on the Au/Pt/Rh interface can provide active hydroxyl species, so that the adsorption of formate and the desorption of generated hydrogen species are promoted at Pt atoms on the Au/Pt/Rh interface. Consequently, Au6Pt1Rh0.5/C electrocatalysts exhibit excellent mass activity (8.05 A mgPt−1) and specific activity (14.3 mA cm−2) for the FAOR, which exceed those of most of the reported Pt-based or Pd-based electrocatalysts.


 Please wait while we load your content...
Please wait while we load your content...