g-C3N4/MgO nanosheets: light-independent, metal-poisoning-free catalysts for the activation of hydrogen peroxide to degrade organics†
Abstract
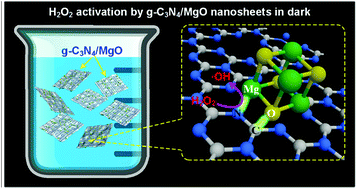
The huge amount of organic wastewater produced by dye manufacturing/consuming industries causes serious environmental pollution, which urges researchers to explore highly efficient and cost-effective Fenton-like catalysts. Herein, we report the synthesis of g-C3N4/MgO nanosheets through a simple two-step calcination method, exhibiting the excellent catalytic ability of activating hydrogen peroxide to degrade organic dyes in the dark. The results of this study indicate that the g-C3N4/MgO nanosheets are light-independent and metal-poisoning-free Fenton-like catalysts for enhanced degradation of organics. The efficient degradation performance is attributed to hydroxyl radical generation in the g-C3N4/MgO–H2O2 catalytic system. The bonding between the MgO and g-C3N4 parts of the g-C3N4/MgO nanosheets is considered to be a key role in activating H2O2 to produce a hydroxyl radical. This catalytic degradation process can be conducted in a wide pH range and at room temperature without external energy input. Additionally, the g-C3N4/MgO nanosheets are non-toxic, low-cost and easy to prepare. Therefore, it is reasonable to believe that g-C3N4/MgO nanosheets have a great potential in the catalytic degradation of organic contaminants and could be used in a wide range of environmental cleanup applications. Furthermore, this work provides an insight into exploiting novel Fenton-like catalysts without transition metal components or external energy excitation.


 Please wait while we load your content...
Please wait while we load your content...