Self-assembled alloy nanoparticles in a layered double perovskite as a fuel oxidation catalyst for solid oxide fuel cells†
Abstract
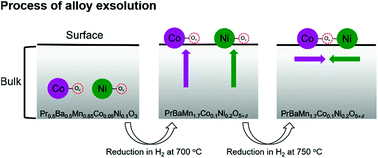
In situ exsolved nanoparticles on metal oxide materials have received much attention in catalysis due to their well socketed structure and high catalytic activity. Recently, the demand for active nanoparticles with multiple functionalities in catalysis has increased, so exsolutions of intermetallic nanoparticles could be an effective strategy to meet the requirements. Herein, for the first time, we report exsolved Co–Ni alloy nanoparticles and their Gibbs free energy of alloy formation in a PrBaMn1.7Co0.1Ni0.2O5+δ layered double perovskite. These exsolved alloy nanoparticles have a high catalytic performance for fuel oxidation in fuel cells and in the dry reforming of methane. Furthermore, we probed the mechanism of the alloy formation in the exsolution using density functional theory (DFT). The theoretical calculations reveal that the Gibbs free energy of the surface alloy formation (ΔGaggr_surface) is more favorable than that of the bulk alloy formation (ΔGaggr_bulk), indicating that Co and Ni are exsolved separately from the bulk, and then aggregate to form a Co–Ni alloy on the surface.


 Please wait while we load your content...
Please wait while we load your content...