Na-ion battery cathode materials prepared by electrochemical ion exchange from alumina-coated Li1+xMn0.54Co0.13Ni0.1+yO2†
Abstract
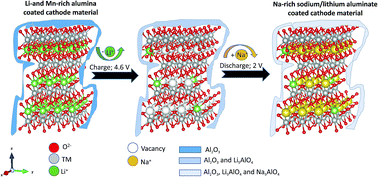
Layered cathode materials of the type Na1+xLi0.05Mn0.54Co0.13NiyO2−z (0 < x < 0.1, y = 0.13 or 0.2, z < 0.1) were prepared by direct ion-exchange reactions, starting from Li1.2Mn0.54Co0.13Ni0.13O2 and Li1.13Mn0.54Co0.13Ni0.2O2. They were examined vs. Na foil or sodiated hard carbon anodes as high capacity positive electrode materials for Na ion batteries with an initial reversible capacity >200 mA h g−1. Analysis by X-ray and electron diffraction reveals that the new materials are initially mixtures of rhombohedral and monoclinic phases. Attempts to prepare layered compounds with similar compositions by chemical means resulted in phases of hexagonal structure with rather poor electrochemical activity, emphasizing the importance of the synthesis by electrochemical ion exchange. Also, the stability of electrochemically prepared Na insertion cathode materials in cycling experiments was insufficient for practical consideration. In turn, cathodes prepared from the same Li precursors coated by a thin layer of alumina via atomic layer deposition, followed by electrochemical Na/Li ion exchange, demonstrated stable capacity (>170 mA h g−1) during prolonged cycling. Their average discharge voltage was 300 mV higher compared to the counterpart uncoated Na intercalation cathodes. The structure and behavior of these electrodes were thoroughly explored by a variety of analytical and surface tools, in conjunction with electrochemical techniques.
- This article is part of the themed collection: Industry R&D collection


 Please wait while we load your content...
Please wait while we load your content...