Bayberry tannin immobilized bovine serum albumin nanospheres: characterization, irradiation stability and selective removal of uranyl ions from radioactive wastewater†
Abstract
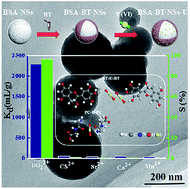
With the widespread use of nuclear energy, uranium containing radioactive waste water has raised global concerns on environmental sustainability, calling for high-performance materials in effective pollution treatments. Here, a novel adsorbent was prepared by immobilizing bayberry tannin onto bovine serum albumin nanospheres (BSA–BT-NSs), which was shaped by antisolvent method. The as-prepared adsorbent was characterized by SEM, TEM, FT-IR, and XPS, and showed excellent adsorption capacity and high selective adsorption for uranyl ions (UO22+) from radioactive wastewater, which included Fe3+, Mn2+, Cs2+, Co2+, and Sr2+. The effects of different adsorption parameters such as pH, temperature, time, and initial UO22+ concentration were investigated. The adsorption isothermal data were well fitted by the Langmuir isotherm model, and a maximum adsorption capacity of 487.805 mg g−1 was estimated. Furthermore, the BSA–BT-NSs possess excellent stability under 60Co γ-ray irradiation at total doses ranging from 10 kGy to 350 kGy. These facts indicated that BSA–BT-NSs could be used as a low-cost and environmentally friendly adsorbent for UO22+ removal with high efficiency and selectivity from various uranium-containing radioactive industrial effluents. More importantly, combined with the characterization analysis, adsorption experimental results, and theory calculations based on density functional theory, the adsorption mechanism of the BSA–BT-NSs is revealed.


 Please wait while we load your content...
Please wait while we load your content...