Thermally stable and coking resistant CoMo alloy-based catalysts as fuel electrodes for solid oxide electrochemical cells†
Abstract
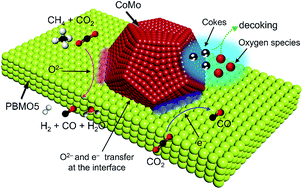
Demands for the development of active, stable nanoparticle-based alloys (NAs) as well as an effective approach to maintaining their nanoscale dimensions at high temperature are rapidly increasing for solid oxide electrochemical cells. While in situ exsolution from perovskite oxides can produce highly active and stable NAs, their composition flexibility is rather poor. Note that only reducible cations, such as Ni, Co, Fe, Cu, and precious metals, can be exsolved. The infiltration technique allows us to develop NAs with new, complex compositions, but the derived NAs are more likely to aggregate into larger particles in comparison to the exsolved NAs. Here we report the synthesis of a novel CoMo NA with a double shell structure. Such a double shell structure may slow down the atomic diffusion rate, and thus benefit the enhancement of the thermal stability of the CoMo NA. In addition, we construct a mosaic-like PrBaMn2O5+δ matrix to modify the Y2O3-stabilized-ZrO2 electrolyte scaffold. This rough, defect-rich matrix layer helps disperse and anchor the CoMo NAs and sets the stage for synergetic catalysis. The composite electrode exhibits good reactivity, robustness and coking resistance in fuel electrodes for hydrocarbon oxidation and CO2 splitting, showing promise to replace the state-of-the-art counterparts. More generally, this work highlights a simple and viable pathway for enhancing the performance of solid oxide electrochemical cells as well as other high temperature (electro)catalysis reactions.


 Please wait while we load your content...
Please wait while we load your content...