Pt/CePO4 catalysts for the WGS reaction: influence of the water-supplier role of the support on the catalytic performance
Abstract
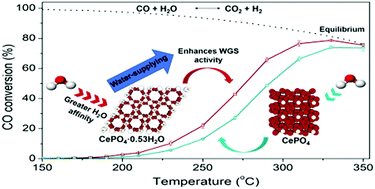
For Pt catalysts which have demonstrated great activity for the WGS reaction, the activation of water is described as the rate-limiting step. Such limitation could be overcome through the design of supports able to supply water. In this study, the hexagonal and monoclinic phases of CePO4 have been evaluated as supports for Pt WGS catalysts. The hexagonal structure presents channels containing water, absent in the monoclinic structure. The presence of these channels in the hexagonal phase increases the interaction with the water molecules, leading to an enhancement of the WGS catalytic performance. DRIFTS results showed that dissociation of water does not occur on these supports, while calculated apparent activation energies present values similar to those reported in the literature for the dissociation of water in Pt (111). These results suggest that cerium phosphates act as water suppliers, increasing the number of available species to be dissociated on the Pt surface.


 Please wait while we load your content...
Please wait while we load your content...