Hierarchical heterostructure NiCo2O4@CoMoO4/NF as an efficient bifunctional electrocatalyst for overall water splitting†
Abstract
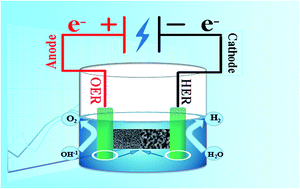
The design of efficient and economical noble-metal-free bifunctional electrocatalysts for both oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) is needed to fulfil the promise of hydrogen production technologies through overall water splitting. Herein, hierarchical NiCo2O4@CoMoO4 on Ni foam (NF) was synthesized via a facile two-step method which involved hydrothermal and calcination processes. In addition, to explore the mechanism for the growth of hierarchical NiCo2O4@CoMoO4/NF nanostructures, variable time-dependent experiments were carried out. The results showed that the optimal NiCo2O4@CoMoO4/NF-7 electrode exhibits superior electrocatalytic performance and excellent durability for OER, HER and overall water splitting, which presents a low overpotential of 265 mV at a current density of 20 mA cm−2 for OER and 121 mV at a current density of 10 mA cm−2 for HER in 1.0 M KOH. Meanwhile, when used as an electrocatalyst electrode for overall water splitting, NiCo2O4@CoMoO4/NF-7 shows a quite low cell voltage of 1.55 V at 10 mA cm−2, and long-term durability during a 12 h stability test at a current density of 10 mA cm−2 in 1.0 M KOH. The study demonstrates that synergistic effects between NiCo2O4 and CoMoO4 in NiCo2O4@CoMoO4/NF heterostructures can effectively enhance electrochemical performance and provides a wide range of possibilities for the further development of inexpensive electrode materials for overall water splitting.


 Please wait while we load your content...
Please wait while we load your content...