Reversible facile Rb+ and K+ ions de/insertion in a KTiOPO4-type RbVPO4F cathode material†
Abstract
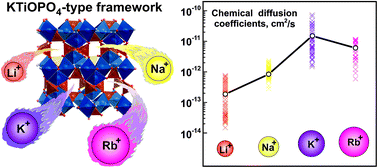
In this paper, we report on a novel RbVPO4F fluoride phosphate, which adopts the KTiOPO4 (KTP) type structure and complements the AVPO4F (A = alkali metal) family of positive electrode (cathode) materials for metal-ion batteries. RbVPO4F was synthesized via a freeze-drying assisted solid-state route and characterized via structural, computational and electrochemical methods. RbVPO4F represents the first example of reversible electrochemical Rb+ de/insertion in a crystalline oxypolyanionic framework. The electrochemical measurements on RbVPO4F in a three-electrode cell configuration in RbClO4-saturated propylene carbonate (PC) electrolyte revealed that the material exhibits reversible Rb+ de/insertion within the 0.4–1.3 V vs. Ag+/Ag potential range (∼3.9–4.8 V vs. K+/K) displaying rather high diffusion coefficients of (0.3–1.0) × 10−11 cm2 s−1 comparable to those of K+ in KVPO4F that supports reasonably fast ionic mobility in the KTP structure despite the large ionic radius of the Rb+ ions. The energy barriers of Rb+ ion transport are exceptionally low not exceeding 0.2 eV along the c-axis and correlating well with diffusion coefficients estimated using the DFT+U-NEB methodology and with the experimentally determined transport properties. These results suggest a new paradigm for the development of materials that support many monovalent ion reversible de/insertion processes in a single prototypical structural oxypolyanionic framework.


 Please wait while we load your content...
Please wait while we load your content...