Kinetically controlled redox behaviors of K0.3MnO2 electrodes for high performance sodium-ion batteries†
Abstract
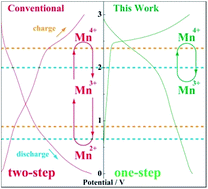
Due to its abundance, high theoretical capacity, and environmental compatibility, manganese dioxide (MnO2) is regarded as a potential electrode material for sodium-ion batteries. Nevertheless, severe side reactions including manganese dissolution and the Mn3+ disproportionation reaction are common challenges to manganese-based electrodes that cause a huge capacity fading, further limiting their practical applications. To address these issues, here the environmentally friendly K-birnessite MnO2 (K0.3MnO2) nanosheets are directly inkjet-printed on the stainless steel sheet to serve as the electrode, while a diglyme-based electrolyte is used to fabricate a sodium-ion battery. In contrast to the conventional two-step redox reactions involving Mn4+/Mn3+/Mn2+ couples, the as-printed K0.3MnO2 electrode shows an enhanced redox activity of the Mn4+/Mn3+ couple, along with a suppressed redox activity of the Mn3+/Mn2+ couple that restricts the side reactions. The active particle size, electrode structure and electrolyte conditions could be identified as the key factors that contribute to the performance optimization. The electrode simultaneously and unprecedentedly achieves a working voltage of 2.5 V, maximum energy and power densities of 587 W h kgcathode−1 and 75 kW kgcathode−1, respectively, with 99.5% capacity retention for 500 cycles at 1 A g−1.


 Please wait while we load your content...
Please wait while we load your content...