A nanosized metal–organic framework with small pores for kinetic xenon separation†
Abstract
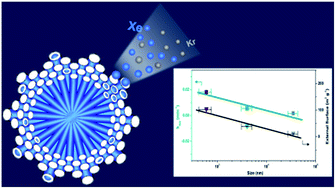
Nanosizing of crystals can bring superior properties into materials, particularly for facilitating mass transport in porous materials. Herein, we report the synthesis of CaSDB metal–organic framework (MOF) nanocrystals using additives; two types of additives (ammonia and lauric acid) are adopted to effectively control the crystal size down to 400–700 nm. In individual synthesis systems, ammonia acts as a nucleation promoter and lauric acid serves as a growth inhibiter during crystallization. The CaSDB crystals can be easily engineered from nanoscale (600 nm) to macroscale (35 μm) by adjusting the ammonia content in the synthetic solutions. The CaSDB nanocrystals prepared using both additives have the same porosity including the same pore size (5.0 Å), similar surface areas (140–157 m2 g−1), and ultramicropore volumes (0.02–0.027 cm3 g−1), probed by N2 sorption measurements. Xe and Kr adsorptions show that the CaSDB nanomaterials exhibit a good thermodynamic selectivity of 20.5 for Xe over Kr owing to the optimized interactions between Xe molecules (4.1 Å) and CaSDB pores (5.0 Å). Breakthrough measurements show that the dynamic selectivity for Xe over Kr is close to the thermodynamic selectivity. More importantly, nanosized CaSDB MOF crystals exhibit a very fast rate for xenon adsorption that is demonstrated by a significantly higher rate constant of nanocrystals (1.8 × 10−2 min−1) than those of micro- (5.53 × 10−3 min−1) and macrocrystals (1.65 × 10−3 min−1). The enhanced adsorption rate is due to the high external surface area of CaSDB nanocrystals. Repetitive tests reveal that the CaSDB nanomaterials are robust and reproducible in terms of Xe/Kr selectivity and Xe uptake and hold great promise for application in adsorption-based xenon separation and capture.


 Please wait while we load your content...
Please wait while we load your content...