Two-step synthesis of Sillén–Aurivillius type oxychlorides to enhance their photocatalytic activity for visible-light-induced water splitting†
Abstract
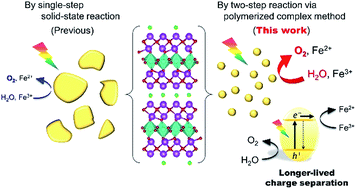
A two-step synthesis via the polymerized complex method (2PC) was developed to improve the photocatalytic activity of a Sillén–Aurivillius oxychloride Bi4TaO8Cl and related oxychlorides for O2 evolution (i.e., water oxidation) in Z-scheme water splitting under visible light. This method uses the polymerized complex reaction to prepare a precursor oxide (e.g., Bi3TaO7), which is subsequently calcined with BiOCl to yield a pure Bi4TaO8Cl phase with smaller particle sizes than those obtained via a conventional single-step solid-state reaction (1SSR). Furthermore, time-resolved microwave conductivity (TRMC) measurements revealed that the Bi4TaO8Cl sample prepared by the 2PC method at 973 K (2PC_973) achieved more than five times longer-lived charge separation than that by the 1SSR at 973 K (1SSR_973), which probably arises from lower numbers of charge-recombination centers produced in the 2PC synthesis. Thus, the synthesized Bi4TaO8Cl samples exhibited a higher rate of O2 evolution (e.g., 20 μmol h−1 for 2PC_973 vs. 4 μmol h−1 for 1SSR_973). Overall water splitting into stoichiometric H2 and O2 was demonstrated by constructing a Z-scheme photocatalytic system consisting of 2PC_973, Ru-modified SrTiO3:Rh, and an Fe3+/Fe2+ shuttle redox mediator, with an external quantum efficiency of 0.9% at 420 nm, which was much higher than that using the sample derived from the optimized 1SSR method at 1173 K (0.4%). The 2PC synthesis was successfully extended to other Sillén–Aurivillius type oxychlorides, Bi4NbO8Cl, Bi6NbWO14Cl and Sr2Bi3Ta2O11Cl, all of which exhibited superior water splitting activity compared to those prepared through the 1SSR.


 Please wait while we load your content...
Please wait while we load your content...