Ring closure of polynitroazoles via an N,N′-alkylene bridge: towards high thermally stable energetic compounds†
Abstract
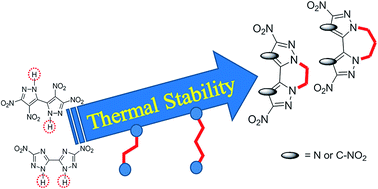
High thermal stability is one of the important factors in the design of high-performance insensitive energetic compounds. A straightforward synthesis of tricyclic energetic molecules (2, 3, 5 and 6) has been developed through bridging ethylene-/propylene-moieties featuring two polynitroazoles. Remarkably, such ring closure reactions lead to higher thermal stability. In addition, the influence of 4,4′,5,5′-tetranitro-2H,2′H-3,3′-bipyrazole (1) and 5,5′-dinitro-2H,2′H-3,3′-bi-1,2,4-triazole (4) with or in the absence of an N,N′-alkylene bridge on the structural and electrostatic potentials (ESP) was established theoretically. The high thermal stability and good detonation properties may make these materials useful as replacements for trinitrotoluene (TNT).


 Please wait while we load your content...
Please wait while we load your content...