Selective and ppb level removal of Hg(ii) from water: synergistic role of graphene oxide and SnS2†
Abstract
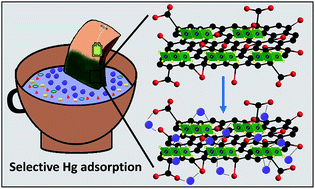
Mercury (Hg) contamination can cause serious health issues like brain damage, skin diseases and birth defects. An upper limit of 2 ppb for Hg in drinking water has been allowed by the United States Environmental Protection Agency (EPA). Thus, the selective removal of Hg below 2 ppb is an important challenge in the treatment of wastewater. Herein, we report the tremendous selectivity and ppb level removal of Hg(II) from water by using a graphene oxide and tin(IV) disulfide (SnS2) composite (GO@SnS2). The material can remove 99.1% of Hg(II) from a concoction of Na(I), K(I), Cs(I), Rb(I), Ca(II), Mg(II), Co(II), Cu(II), Ni(II), Zn(II), Pb(II), Cd(II), Mn(II), Fe(III) and As(III) with a high separation factor of the order 102 to 103. We have achieved a capacity of 342.02 ± 8.02 mg g−1 with a distribution coefficient (Kd) value of 8.68 × 105 mL g−1 and GO@SnS2 is stable in the pH range of 0.5–11. The material can remove Hg(II) from even 0.3 ppb Hg(II) contaminated water. Furthermore, the mechanism behind the synergistic Hg(II) adsorption is due to the interaction between Hg(II) and –COOH of GO and the soft Lewis acid-base chemistry between S2− of SnS2 and Hg(II). For convenient application, we have designed a tea bag filled with GO@SnS2 powder which can capture 99.9% of Hg(II) from contaminated water economically.


 Please wait while we load your content...
Please wait while we load your content...