Rational design of Na(Li1/3Mn1/2Cr1/6)O2 exhibiting cation–anion-coupled redox reactions with superior electrochemical, thermodynamic, atomic, and chemomechanical properties for advanced sodium-ion batteries†
Abstract
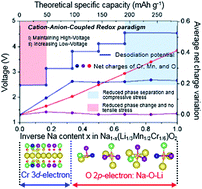
Anionic redox reactions (O2−/O−), an alternative to conventional cationic redox reactions (Mn+/M(n+1)+; M: transition metal), have recently been identified as essential to achieve high energy density cathodes for sodium-ion batteries (SIBs). To overcome the drawbacks of anionic redox reactions leading to phase change and separation in the newly discovered Na(Li1/3Mn2/3)O2 material (NLMO, ∼4.2 V vs. Na/Na+ with a high charge capacity of 190 mAh g−1), we have rationally designed high energy density Na(Li1/3Mn1/2Cr1/6)O2 (NLMCO) in which the Cr 3d-electron is coupled with the labile O 2p-electron coordinated with Mn4+ for charge compensation during desodiation processes. NLMCO exhibits reduced phase change and separation, and chemomechanical strain and stress compared to NLMO and is thus expected to show high electrochemical performance, where the formation of short O–O bonds is not observed. By correlating the thermodynamic energy behavior with the redox mechanism in NLMO, it is concluded that our systematically designed cation–anion-coupled NLMCO is an excellent cathode material, introducing advanced materials of formula Na(Li1/3M2/3(1−y)Mcy)O2 (M and Mc: transition metals with stabilized M4+ species and cationic redox active Mc4+ species) for next-generation SIBs.


 Please wait while we load your content...
Please wait while we load your content...