Synthesis of hierarchical structured Fe2O3 rod clusters with numerous empty nanovoids via the Kirkendall effect and their electrochemical properties for lithium-ion storage†
Abstract
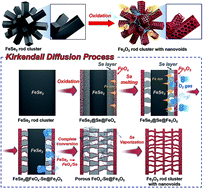
The Kirkendall effect has been widely applied to prepare hollow/porous metal-oxide-based composites. We propose a new mechanism for the transformation of hierarchical structured metal selenides into their corresponding metal oxides with unique structures via the Kirkendall effect. Based on this mechanism, hierarchical structured iron oxide clusters comprising one-dimensional nanorods with numerous empty nanovoids were successfully prepared. FeSe2 rod clusters synthesized by a one-pot hydrothermal process were post-treated under an air atmosphere. During the oxidation process, FeSe2@FeOx–Se@Fe2O3 and FeOx–Se@Fe2O3 intermediates are formed owing to the different diffusion rates of iron cations, the selenium component, and oxygen gas. As the oxidation proceeded, elimination by evaporation of the SeO2 layer formed by the reaction of the diffused-out metalloid Se and oxygen gas and oxidation of FeOx resulted in porous Fe2O3 nanorods with numerous interconnected empty nanovoids. When employed as a lithium-ion battery anode, hierarchical Fe2O3 rod clusters exhibited high reversible discharge capacity, good cycling stability, and excellent rate performance. Following 200 cycles, their discharge capacity is 1318 mA h g−1 at a current density of 1 A g−1. Additionally, the rod clusters delivered a discharge capacity of 745 mA h g−1 at a high current density of 10 A g−1.


 Please wait while we load your content...
Please wait while we load your content...