The synergetic effect of h-BN shells and subsurface B in CoBx@h-BN nanocatalysts for enhanced oxygen evolution reactions†
Abstract
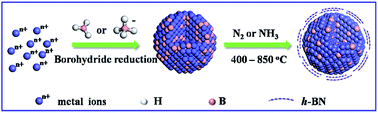
Boron-containing cobalt (Co–B) nanoparticles (NPs) synthesized by the borohydride reduction process were subjected to ammonization. Part of B species segregate onto particle surfaces to form ultrathin h-BN shells which stabilize highly dispersed Co NPs under harsh conditions and the remaining B atoms interact strongly with Co atoms inside the NPs. The formed CoBx@h-BN core–shell nanocatalysts exhibit an overpotential of 290 mV at a current density of 10 mA cm−2 making them among the most active catalysts for the oxygen evolution reaction (OER) and they present 150 h OER stability. The improved OER performance has been attributed to the synergetic role of the confinement effect of h-BN shells and activation effect of subsurface B atoms. This work suggests a simple synthetic route to prepare highly active and stable metal nanocatalysts without using any surfactants and supports.


 Please wait while we load your content...
Please wait while we load your content...