C2N-supported single metal ion catalysts for HCOOH dehydrogenation†
Abstract
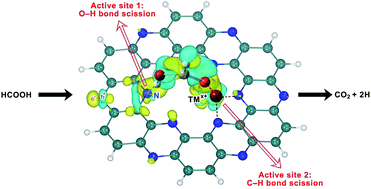
High catalytic performance of a single-atom transition metal ion (TMx+) anchored on the two-dimensional (2D) C2N lattice is predicted for HCOOH dehydrogenation. Considering the Co2+, Cu2+ and Ni2+ non-noble metal ions supported by C2N, we use density functional theory to demonstrate dehydrogenation energy barriers as low as those for pure Pt and Pd catalysts. The high catalytic performance is ascribed to the reaction occurring through a dual-active center composed of TMx+ and a nearby N atom of C2N. Specifically, C2N–Co2+ in the low spin state (S = 1/2) greatly promotes HCOOH dehydrogenation by decreasing the barrier of the rate-determining step to only 0.30 eV, mainly due to the strong ability of TMx+ to extract charges from HCOOH and C2N. The obtained mechanistic insights help the rational design of single-atom based transition metal ion catalysts supported by 2D materials.


 Please wait while we load your content...
Please wait while we load your content...