Surface plasmon-enhanced activity and stability for methanol oxidation on gold caviar-like assembly under solar light†
Abstract
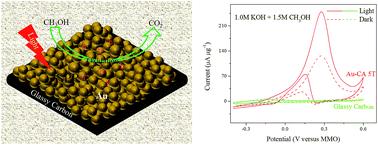
The surface plasmon-enhanced catalytic performance of a gold caviar-like assembly (Au-CA) in methanol electrooxidation was investigated with and without simulated solar irradiation. An Au-CA catalyst synthesized by an ion sputtering method not only exhibited an excellent anodic peak current density (115.86 μA μg−1) but also displayed long-term catalytic stability for methanol electrooxidation. The Au-CA catalyst also provided an anodic peak current density of 248.9 μA μg−1 for methanol electrooxidation under simulated solar irradiation. This value corresponded to a 2.15-fold increase with respect to that in the absence of solar light irradiation in a deoxygenated solution of 1.0 M KOH and 1.5 M CH3OH. The diffuse reflectance UV-vis absorption spectra and photoelectric response performance of the as-prepared Au-CA catalyst were investigated to understand the effectiveness of the surface plasmon resonance of Au nanoparticles for the enhancement of methanol electrooxidation performance. The results showed that the Au-CA catalyst exhibited a broad absorption peak at a wavelength of 515 nm with a red shift in comparison with that of a solution of Au nanoparticles. The photocurrent density of the Au-CA catalyst was 175.8 μA μg−1 at an irradiation wavelength of 468 nm, which was 2.5 times higher than that at 640 nm (70.2 μA μg−1) in a deoxygenated solution of 0.5 M Na2SO4. These findings suggested the potential of the new strategy for the improvement of the activity and durability of Au in methanol electrooxidation in direct methanol fuel cell technologies.


 Please wait while we load your content...
Please wait while we load your content...