A multitude of modifications strategy of ZnFe2O4 nanorod photoanodes for enhanced photoelectrochemical water splitting activity†
Abstract
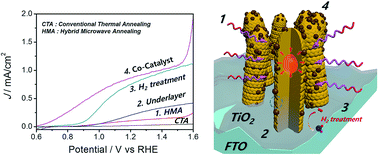
Numerous modifications strategies are applied to spinel ZnFe2O4 nanorods with a band gap energy of ∼2.0 eV to enhance their activity as a photoanode for photoelectrochemical (PEC) water splitting. First, hybrid microwave annealing (HMA) imparts high crystallinity to ZnFe2O4 nanorods, while preserving the formed nanostructure and maintaining high electric conductivity of F:SnO2 (FTO) substrate. This is in contrast to conventional thermal annealing (CTA) at 800 °C that causes aggregation of ZnFe2O4 and degradation of FTO. Second, insertion of a TiO2 underlayer blocks charge recombination at the FTO/electrolyte interface and serves as a source of Ti doping. Third, hydrogen treatment yields oxygen vacancies that increase charge carrier density and cause surface passivation. Last, a NiFeOx co-catalyst promotes hole injection into the electrolyte to improve catalytic water oxidation activity. These synergistic modifications lead to enhanced photocurrent density from 0.025 mA cm−2 at 1.23 VRHE for pristine ZnFe2O4 nanorods prepared by CTA to 0.92 mA cm−2 for a fully modified HMA photoanode: a 37-fold increase in photocurrent density. There is also a cathodic shift of the onset potential down to 0.62 VRHE. The multiple modifications enhance bulk charge separation efficiencies from mere 2% to 30% and surface charge separation efficiency from 40% to 80%.


 Please wait while we load your content...
Please wait while we load your content...