Intensification of anodic charge transfer by contaminant degradation for efficient H2 production†
Abstract
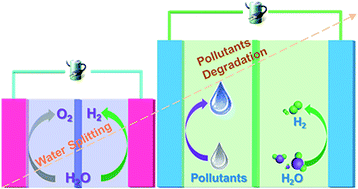
Hydrogen (H2) production from electrocatalytic water splitting is at present limited by the sluggish oxygen evolution reaction (OER). Breakthroughs in low overpotential and stable anodic reactions are urgently needed to complement the rapid advancements in H2 production. Using nickel (Ni)-based catalysts as an example, we intensified charge transfer on a nickel phosphide (Ni2P) anode for efficient H2 production using substitution of contaminant degradation for the OER. Based on X-ray photoelectron spectroscopy (XPS) and in situ Raman spectroscopy, we confirmed that a decreased two-electron-transfer pathway was responsible for the superior formate oxidation, whereas the formation of the desired oxidation state of NiIII accounted for the efficient urea degradation. Both scenarios were beneficial for accelerating charge transfer across the interface between the anode and electrolyte, thus achieving high H2 production efficiency at low voltage. A voltage of only 1.40 V supported currents of 12.5 and 6.0 mA in urea- and formate-containing solutions, respectively, which reached 150 and 60 mA after the voltage was increased to 1.70 V, several times higher than that under basic conditions.


 Please wait while we load your content...
Please wait while we load your content...