Direct observation of cation-exchange in liquid-to-solid phase transformation in FA1−xMAxPbI3 based perovskite solar cells†
Abstract
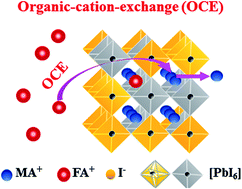
Mixed-organic-cation FA1−xMAxPbI3 perovskites are currently attracting intensive attention for their high photovoltaic performance. However, FA1−xMAxPbI3 usually has an undesired non-perovskite phase leading to a compromised efficiency, and a facile strategy to achieve pure-phase FA1−xMAxPbI3 has been rarely reported. Here, we demonstrate a facile approach to form δ-phase free FA1−xMAxPbI3 using a non-stoichiometric precursor solution. It is found that a small amount of excess methylammonium iodide (MAI) added to the precursor solution has a profound effect on perovskite crystallization during the liquid-to-solid phase transformation. Using an in situ photoluminescence spectroscopy measurement, it is found that the excess MAI can promote the formation of FA1−xMAxPbI3 and facilitate the cation-exchange between organic FA+ and MA+ during the film formation. Based on the optimized pure-phase FA1−xMAxPbI3, the perovskite solar cells exhibit an encouraging power conversion efficiency (PCE) of 17.40%. This study demonstrates a facile method to achieve high performance FA dominating mixed-organic-cation perovskite devices while providing insight into the ion-exchange process during perovskite crystallization.


 Please wait while we load your content...
Please wait while we load your content...