Solid polymer electrolytes from a fluorinated copolymer bearing cyclic carbonate pendant groups†
Abstract
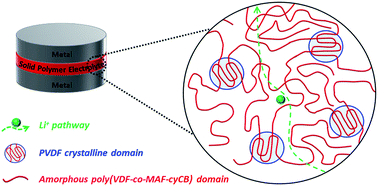
A poly(vinylidene fluoride-co-(2-oxo-1,3-dioxolan-4-yl)methyl 2-(trifluoromethyl)acrylate) random copolymer, poly(VDF-co-MAF-cyCB), with a MAF-cyCB weight fraction of 59% was synthesized via free radical copolymerization of VDF and MAF-cyCB, which is a methacrylate bearing cyclocarbonate side-chain. This copolymer showed nano-structured morphology, where crystalline PVDF-rich domains co-existed with amorphous poly(VDF-co-MAF-cyCB) segments. Solid polymer electrolytes were further obtained by loading the poly(VDF-co-MAF-cyCB) copolymer with various amounts of LiClO4. The added lithium salt was dissolved in the poly(VDF-co-MAF-cyCB) amorphous phase, which allowed the formation of an ionic conducting phase exhibiting ionic conductivity values as high as 2 × 10−4 S cm−1 at room temperature for an optimum cyCB/Li+ molar ratio of 5. The addition of LiClO4 up to the optimum cyCB/Li+ molar ratio of 5 also increased the phase separation between the crystalline and amorphous phases, the mechanical properties of the material (up to 107 at 102 rad s−1) and the ionic conductivity (>10−3 S cm−1 at 80 °C). Furthermore, an electrochemical stability window from 1.4 to 4.9 V vs. Li/Li+ and relatively high values for the measured lithium ions transference numbers (0.68 at 40 °C) were observed, making the investigated system a promising candidate for next generation solid polymer electrolytes.


 Please wait while we load your content...
Please wait while we load your content...