A Co–N4 moiety embedded into graphene as an efficient single-atom-catalyst for NO electrochemical reduction: a computational study†
Abstract
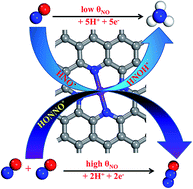
Electrochemical reduction of nitric oxide (NOER) is a promising technology for the removal of harmful N-containing species in groundwater under mild conditions. In this work, by means of density functional theory computations, we systematically investigated the potential of utilizing experimentally feasible transition metal–N4/graphenes as NOER catalysts. Our results revealed that NO molecules can be moderately activated on a Co–N4 moiety embedded into graphene, and the subsequent NOER steps can proceed to form either NH3 at low coverages or N2O at higher coverages. Especially, the computed onset potential of NOER on Co–N4/graphene (ca. −0.12 V) is comparable to (or even better than) those on well-established Pt-based catalysts. Thus, Co–N4/graphene is a promising single-atom-catalyst with high efficiency for NO electrochemical reduction, which opens a new avenue for NO reduction for environmental remediation.
- This article is part of the themed collection: International Year of the Periodic Table : Single Atoms as Active Catalysts


 Please wait while we load your content...
Please wait while we load your content...