Phonon glass behavior beyond traditional cage structures: synthesis, crystal and electronic structure, and properties of KMg4Sb3†
Abstract
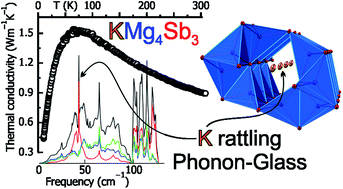
Three new ternary antimonides, KMg4Sb3 and A2Mg5Sb4 (A = Rb, Cs), were synthesized via high-temperature solid-state reactions and their crystal structures were determined by single crystal X-ray diffraction. All three compounds feature three-dimensional anionic frameworks composed of edge-shared MgSb4 tetrahedra with channels trapping the alkali metal cations. KMg4Sb3 crystallizes in β-BaCu4S3 structure type (Pearson symbol oS32) while Rb2Mg5Sb4 and Cs2Mg5Sb4 are isostructural and crystallize in K2Zn5As4 structure type (Pearson symbol oC44). Band structure calculations predict KMg4Sb3 to be a direct bandgap semiconductor with Eg of ∼1 eV. Characterizations of the transport properties indicate that KMg4Sb3 is a semiconductor with impurity levels. KMg4Sb3 exhibits ultralow total thermal conductivity of 0.9 W m−1 K−1 at 300 K. Potassium cations in the structure of KMg4Sb3 exhibit abnormally large anisotropic displacement parameters at 90 K, a behavior typical for rattling cations. Calculations of the phonon dispersions and density of states support the K rattling as an important contributor to overall thermal conductivity reduction. A Phonon-Glass thermal behavior with K atoms rattling in open channels of Mg4Sb3 framework shines new light on designing low thermal conductivity materials.


 Please wait while we load your content...
Please wait while we load your content...