Dual hydrophobic grafted chains facilitating quaternary ammonium aggregations of hydroxide conducting polymers: a theoretical and experimental investigation†
Abstract
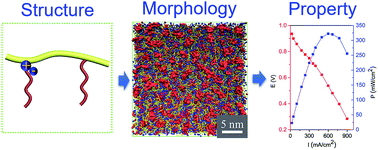
Establishment of connective hydroxide conducting channels is highly desired for alkaline anion exchange membranes (AAEMs). Herein, we offer a feasible strategy of grafting long alkyl chains onto both the quaternary ammonium cation (QA) center and backbone to achieve the formation of well-developed pathways for OH− transport. Theoretical simulations reveal that QA groups are prone to aggregation driven by the thermodynamic incompatibility between the backbone and grafted chains. With increasing length of alkyl chains, micro-phase separation is facilitated. Experimentally, the C16C6-X40Y10 AAEM having the largest population of the longest alkyl chains in this work gives rise to the highest hydroxide conductivity of 61 mS cm−1 at 30 °C, although its water uptake is only 15.8%. The alkaline anion exchange membrane fuel cell with the incorporation of C16C6-X40Y10 yields a maximum power density of 322 mW cm−2 at 60 °C. These results represent the advancements of this dual grafted structure in improving hydroxide conductivities, and this configuration can act as a general platform for developing promising AAEMs in the future.


 Please wait while we load your content...
Please wait while we load your content...