Uncovering the reaction mechanism initiating the nucleation of lead sulfide quantum dots in a hines synthesis†
Abstract
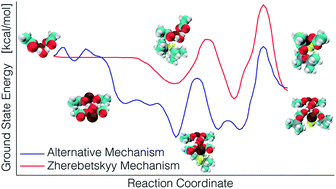
Lead sulfide quantum dots remain the subject of considerable research interest due to their tunable quantum confinement, which leads to exciting photovoltaic and energy storage applications. A major advantage arises from their low-cost fabrication by solution processing. High quality quantum dots can be synthesized in small quantities via colloidal synthesis. However, despite remarkable advances in the synthesis of highly monodisperse dots with precisely programmable structure and composition, details of the mechanism involved in the transformation of molecular precursors to nanoscale crystals remains poorly understood. Surprisingly little is known about the early stages of nucleation, including the stoichiometry, structure, or crystallinity of the hypothesized critical nucleus. Notably, these questions are beyond today's experimental capabilities; the best current technique, in situ X-ray scattering, still requires theoretical models to invert the experimental data. Using accurate Density Functional Theory (DFT), coupled with the Nudged Elastic Band (NEB) method for energy barrier construction, we have analyzed a previously posited reaction mechanism, uncovered some energetically unfavorable aspects, and discovered a new reaction mechanism with a lower energy pathway to PbS quantum dot formation. This new mechanism validates experimental results by Zherebetskyy et al. who revealed the important presence of water molecules in these systems. We provide evidence that the growth of PbS dots occurs in a polymerization-like process, rather than the reactants-to-surface mechanism proposed in earlier work. We also uncover the significant role played by a hydrogen-bonded dimer of lead carboxylate hydrate, which forms in non-polar solvents.


 Please wait while we load your content...
Please wait while we load your content...