Boosting hydrogen evolution via optimized hydrogen adsorption at the interface of CoP3 and Ni2P†
Abstract
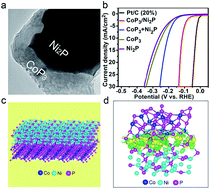
Transition metal phosphides (TMPs) have emerged as highly active catalysts for the hydrogen evolution reaction (HER). Herein, we report a novel excellent CoP3/Ni2P catalyst for the HER with a small onset potential of 51 mV vs. RHE, a low Tafel slope of 49 mV dec−1, a small over-potential value of 115 mV at 10 mA cm−2, and outstanding long-term stability. The normalized polarization curve also shows that CoP3/Ni2P has the best intrinsic catalytic activity compared to pure CoP3 and pure Ni2P. Density functional theory (DFT) calculations reveal that the remarkably enhanced catalytic activity is due to the interface effect of CoP3/Ni2P. The strong interactions at the interface can optimize the electronic environment around the active sites, leading to suitable H+ adsorption and H2 formation kinetics and energetics, thereby enhancing the catalytic activity.


 Please wait while we load your content...
Please wait while we load your content...