A noble-metal-free nanocatalyst for highly efficient and complete hydrogen evolution from N2H4BH3†
Abstract
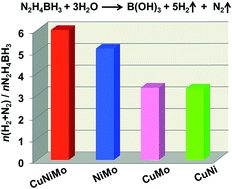
Hydrazine borane (N2H4BH3, 15.4 wt% H) has been considered as a highly promising hydrogen storage material because of its inherent advantages such as high hydrogen content and high stability in the solid state. However, the practical application of N2H4BH3 for the generation of hydrogen is strongly inhibited by the need for expensive noble metal-based catalysts. To overcome this challenge, noble-metal-free CuNiMo nanocatalysts were prepared using a facile chemical reduction approach under an ambient atmosphere at room temperature. Unexpectedly, the resultant CuNiMo catalyst exhibits excellent catalytic activity, and 100% H2 selectivity toward hydrogen generation from N2H4BH3via its BH3 group hydrolysis and N2H4 moiety decomposition at 323 K. To the best of our knowledge, this is the first report of a noble-metal-free catalyst achieving a complete conversion of N2H4BH3 to H2. In addition, CuNiMo can achieve a complete dehydrogenation of hydrous hydrazine (N2H4·H2O). The excellent catalytic performance of the Cu0.4Ni0.6Mo catalyst may be attributed to the electronic modification among Cu, Ni and Mo, and may also be related to the strong basic sites of Cu0.4Ni0.6Mo. The present simple, low cost, highly efficient, and highly selective catalyst may promote the practical application of N2H4BH3 as an effective hydrogen storage material.


 Please wait while we load your content...
Please wait while we load your content...