Bimetallic Ni–Fe phosphide nanocomposites with a controlled architecture and composition enabling highly efficient electrochemical water oxidation†
Abstract
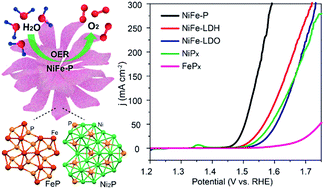
Developing cost-effective and earth-abundant noble-metal-free oxygen evolution reaction (OER) electrocatalysts with high activity and durability is highly desirable for future renewable energy systems. Herein, we develop a facile and controllable approach to engineer a series of structurally tiered bimetallic Ni–Fe phosphide nanocomposites with well-designed architectures and compositions through the topological conversion of the flowerlike precursor of Ni–Fe layered double hydroxides (LDHs). Remarkably, benefitting from their special physicochemical features and desirable synergistic effect, the resultant bimetallic phosphides can serve as a class of advanced high-performance electrocatalysts for water oxidation with an extremely low overpotential of 233 mV to deliver a current density of 10 mA cm−2, a small Tafel slope of 42.5 mV dec−1, and strong durability (30 h of continuous water electrolysis), favourably representing the state-of-the-art level. This work elucidates the promising electrocatalytic performance of multinary transition-metal phosphides with controlled structures and compositions derived from LDHs for the OER.


 Please wait while we load your content...
Please wait while we load your content...