Unravelling the structural and dynamical complexity of the equilibrium liquid grain-binding layer in highly conductive organic crystalline electrolytes†
Abstract
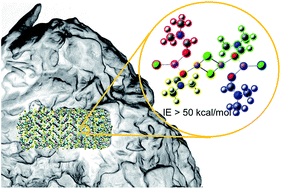
Recent developments in organic crystalline electrolytes for lithium and sodium ion conduction have demonstrated bulk conductivities in the range of 10−4 S cm−1 with negligible grain boundary resistance. Experimental results from EM, XRD, and DSC point to a liquid boundary layer at the crystalline surface in equilibrium with the bulk solid that conducts ions between the grains. In this report we examine this behavior in the electrolyte DMF·LiCl (DMF = N,N-dimethylformamide), which has a bulk conductivity of 1.6 × 10−4 S cm−1, but which decomposes between 360–380 K. Molecular dynamics simulations predict a number of quantitative parameters consistent with experimental observation, such as decomposition temperature (Td(theor) = 380 K, Td(obs) = 360 K), bulk conductivity (σheor = 7 × 10−4, σobs = 1.6 × 10−4) and density (dtheor = 1.209 g mL−1, dobs = 1.306 g mL−1). Further, a number of qualitative properties of the material are predicted by simulation, namely, the crystal packing arrangement, the mechanism of decomposition by expulsion of DMF from the LiCl lattice, the existence of a liquid-like grain boundary layer, and most importantly, negligible grain boundary resistance from increased mobility of ions in the boundary layer vs. the bulk. Finally, from quantum mechanical calculations, various interaction energies between fragmental components explain lattice stability and decomposition of the co-crystal, and highlight the contributions from various possible small aggregates. The theoretical calculations predict decomposition of smaller aggregates, such as those expected in the liquid-like surface, to be more facile than larger aggregates that are more likely to be found in the crystal interior.


 Please wait while we load your content...
Please wait while we load your content...