In situ diffraction studies on reversible oxygen uptake and release in AFe2O4 + δ (A= Lu, Yb, Y, and In)†
Abstract
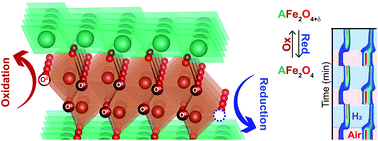
We have studied the reversible uptake and release of oxygen in the layered metal oxide system AB2O4 for A = Lu, Yb, Y, and In to understand their suitability as oxygen storage materials. We first examined their structures in their pristine state of AFe2O4 with high-resolution synchrotron X-ray diffraction. To emulate chemical looping conditions, we then monitored their structures and reactivity under H2 and O2 atmospheres utilizing in situ synchrotron X-ray diffraction and thermogravimetric analysis (TGA) measurements. The nature of the trivalent A cation affects the oxidation kinetics, thermal cycling stabilities, and oxygen storage capacities which varied between 2.20 and 3.13 wt% (0.7–0.98 O2 mmol g−1), corresponding to an oxygen non-stoichiometry (δ) of ∼0.5. These layered oxides underwent various phase transitions above 200 °C which included the creation of a superstructure as oxygen is incorporated until the maximally oxidized phase is established above ∼400 °C. Bond valence sum analysis of the Fe–O bonding across the series reveals that the more underbonded the Fe cation, the more facile the oxygen insertion. During the cycling experiments all samples exhibited stable reversible oxygen insertion at 600 °C with the exception of A = In, which degraded under H2. The Y analogue displayed the fastest kinetics for oxidation, which may make it the most suitable for oxygen storage and sensing applications.


 Please wait while we load your content...
Please wait while we load your content...